Recently, Li Weixue, a researcher and PhD student in the theoretical catalysis research group of the State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences The crystal structure of the catalyst has a decisive influence on the dissociation activity and dissociation path of the carbon monoxide C = O bond, and a clear microscopic mechanism is given. On this basis, the specific synthesis direction of the high specific mass activity and stable cobalt catalyst is predicted. The main research results were published in the Journal of the American Chemical Society (J. Am. Chem. Soc. 135 (2013) 16284–16287) in the form of communication.
The structural sensitivity of the catalytic reaction and the stability of high specific mass active catalytic materials are the key basic scientific issues in the study of heterogeneous catalysis. One of the most representative examples is the use of cobalt catalysts to syngas (carbon monoxide and carbon monoxide) by Fischer-Tropsch synthesis. The mixture of hydrogen) is converted into oil and chemicals. For a long time, it has been found that the syngas conversion activity significantly depends on the crystal phase structure of the cobalt catalyst: when the content of HCP Co in the hexagonal close-packed crystalline phase is higher, the catalytic activity is higher, while when the content of the face-centered cubic close-packed FCC Co is higher Activity is relatively low. Since the actually prepared catalytic materials often contain two crystal phases at the same time, whether HCP Co has higher Fischer-Tropsch intrinsic activity than FCC Co is still an open question. At the same time, as the size of the cobalt catalytic material decreases and the reaction temperature increases, the HCP Co will undergo a structural phase change to FCC Co. The existence of these problems limits the further optimization design research of high-efficiency and stable cobalt catalytic materials.
In response to this problem, Li Weixue's team conducted a detailed study using the first-principles kinetic theory calculations and the activation of carbon monoxide C = O bonds under hydrogen atmosphere as a probe. O-bond activation has a decisive effect: (1) Compared with FCC Co, HCP Co catalyst has higher intrinsic activity, and its catalytic activity also shows a more significant morphological effect; (2) HCP Co tends to directly The C = O bond is dissociated, while FCC Co tends to dissociate the C = O bond indirectly by hydrogenation. Theoretical analysis found that the reason for these differences comes from the relatively low symmetry of the HCP Co crystal structure, which can expose a large number of highly active crystal planes. Based on these results, Li Weixue's team proposed that through the controlled synthesis of HCP Co morphology, specific high-activity HCP Co (10-11) crystal planes were exposed to increase the active site density without the need to reduce the size of the catalyst to achieve high Optimized design of specific mass active, stable cobalt-based catalyst. The results of this study revealed that different crystal phase structures of the material can be used to control the surface structure and morphology, and that the efficient and stable catalytic materials can be obtained by increasing the intrinsic activity and active site density. And the controlled synthesis of materials will play an important role in it.
Li Weixue's research team has long been devoted to systematic theoretical research on the conversion of syngas, and has made good progress in the past: (1) It has been found that the lattice mismatch effect of platinum and cobalt can be used to change the dissociation of the C = O bond on the cobalt catalyst The activated transition state structure reduces the energy barrier of the reaction and improves the activity of the Fischer-Tropsch reaction (J. Am. Chem. Soc. 135 (2013) 4149-4158); (2) It is found that formyl is an important intermediate. Rhodium and cobalt-based catalysts not only play an important role in carbon chain growth, but also in the formation of oxides (Angew. Chem. Int. Ed 50 (2011) 5335-5338). In terms of theoretical research on the stability of catalytic materials, a kinetic equation that completely describes the Ostwald ripening process of nanocatalyst materials loaded under reaction conditions has been established recently, which provides an important research method for inhibiting the rate of the corresponding sintering process (J. Am. Chem . Soc. 135 (2013) 1760-1771).
The above research was supported by the National Natural Science Foundation of China "Outstanding Youth Fund" and the Ministry of Science and Technology "973" project.
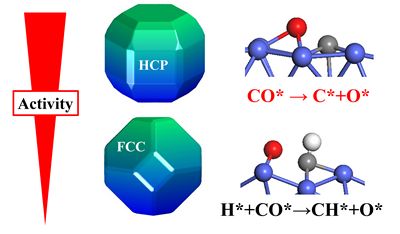
Progress in Research on Crystal Phase Controlling Carbon Oxygen Bond Activation
Zhejiang Shengfa Textiles Printing&Dyeing.,ltd , https://www.chzjsf.com